India
- iDSI has built strong and fruitful collaborative partnerships with government, clinical and academic partners in India through State and Union level, since 2009. Together we have supported India’s ambitious move towards Universal Health Coverage for its 1.3bn population, through strengthening evidence-based decision making in health.
Our Impact
iDSI has:
Provided technical, operational and strategic support to HTAIn, India’s first national Health Technology Assessment (HTA) agency since inception
Delivered comprehensive capacity building in cost-effectiveness analysis for HTAIn and its network of technical collaborators
Supported development of key methodological and technical resources, including the HTAIn reference case and the first National Costing Database
Supported the development of 12 Standard Treatment Guidelines and quality standards, and the first standardised methods manual for guideline adaptation
iDSI initially provided technical assistance to improve Maternal and Child Health at the State level, then in the development of Standard Treatment Guidelines (STGs) and quality standards (QS) at the federal level and supporting their implementation in States. iDSI is presently the primary partner assisting the Ministry of Health and Family Welfare in their efforts to institutionalise the first national body mandated to undertake HTA, building human and organisational capacity in the generation, synthesis and uptake of HTA evidence across the 36 States and Union territories including within Ayushman Bharat and State-level health insurance schemes. This will lead to more transparent and cost-effective health policies and fairer access to high quality health services for patients across India.
Supporting the HTAIn, a made-in-India Health Technology Assessment agency
Impact
The first HTA to be produced by HTAIn with the support of iDSI, intraocular lens for cataract surgery, has already been utilised to inform package inclusion and reimbursement under Ayushman Bharat (Pradhan Mantri Jan Arogya Yojana – PMJAY), the country’s largest health insurance scheme for 500 million Indian citizens – demonstrating rapid and meaningful uptake of HTA evidence into the system and setting a strong precedent for evidence-informed priority setting in India. HTA is now recognised in all National Health Policy Strategy documents and the Government has dedicated significant domestic funding towards HTA-related activity, including funding for over 30 full-time staff members dedicated to HTA in the Central HTAIn Secretariat and six Regional Resource Hubs.
Key reads
iDSI reports
- Supporting HTA capacity building in India: Collaborating to improve HTA, health data and health care across India: Nov 2019
- Institutionalising health technology assessment: establishing the Medical Technology Assessment Board in India
- Identification of publicly available data sources to inform the conduct of Health Technology Assessment in India
- Strengthening health technology assessment systems in the global south: a comparative analysis of the HTA journeys of China, India and South Africa
External resources
- Health Technology Assessment in India (HTAIn) webpage
- Budgeting for a billion: applying health technology assessment (HTA) for universal health coverage in India
- Criteria Used for Priority-Setting for Public Health Resource Allocation in Low- and Middle-Income Countries: A Systematic Review
News
Developing Standard Treatment Guidelines (STGs) to enhance the provision and increase of access to cost-effective, high-quality care
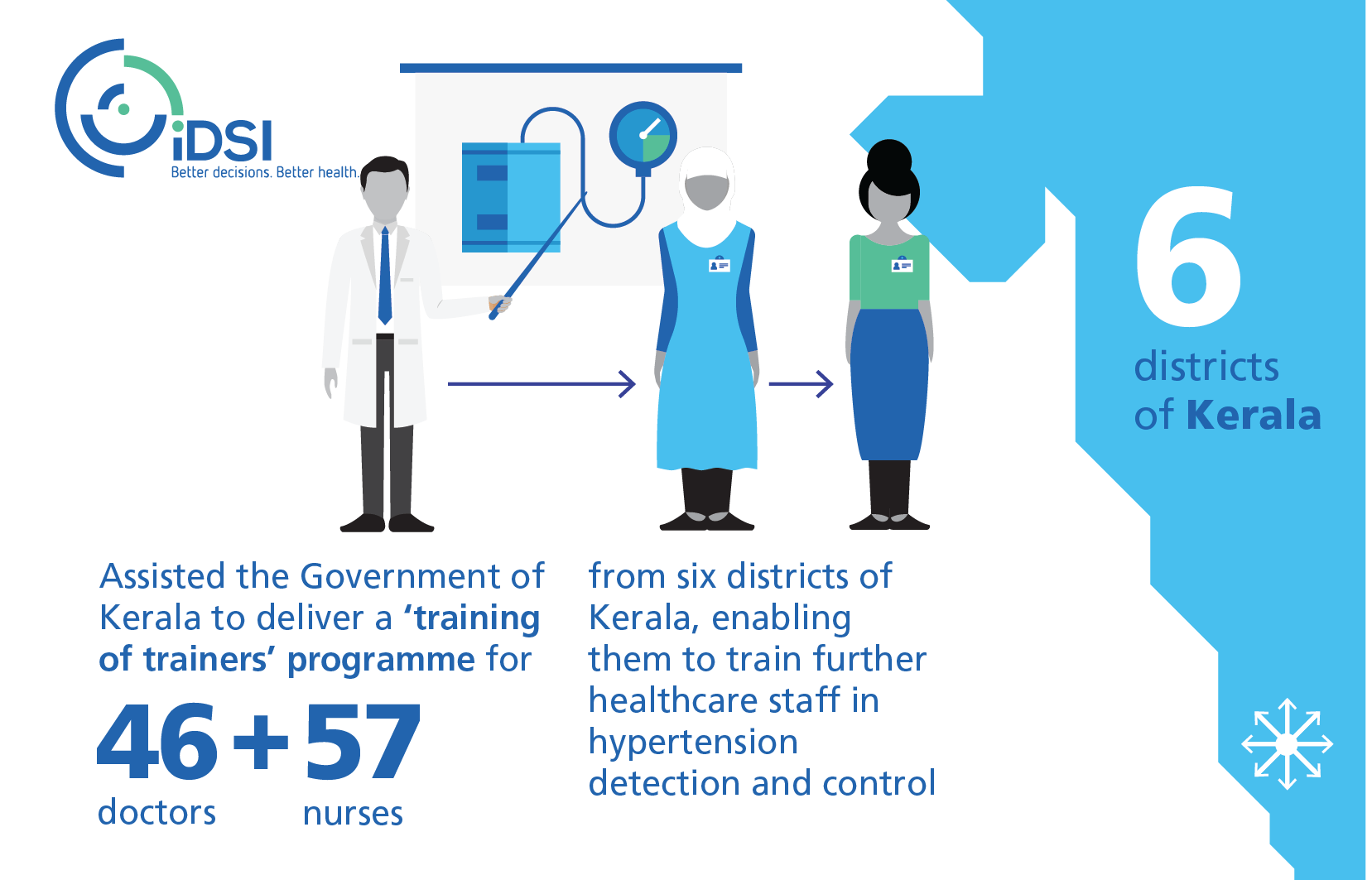
The State Government of Kerala, with technical assistance from iDSI, is now planning to implement quality standards (QS) which are measurable prioritised recommendations from the STG on hypertension. The QS aims to identify undetected cases of hypertension through opportunistic blood pressure measurement for adults visiting a primary healthcare centre. It also aims to improve blood pressure control in known patients. The State Government of Kerala will be integrating indicators for hypertension into the Aadhar linked electronic medical records.
Impact
Modelling estimates found that successful implementation of this guideline (hypertension screening) could prevent up to 966 cardiac events and provide cost savings of US$1.23m for each primary healthcare centre. Extrapolating this across all 28,863 primary healthcare centres in India, under the assumption that only 10% of these benefits could be replicable elsewhere, this could lead to potential cost savings totalling US$3.55bn and the prevention of a total of 2.79 million cardiac events.
Key reads
iDSI resources
Mehndiratta, A., Sharma, S., Gupta, N.P, Sankar, M.J., Cluzeau, F. (2017) Adapting clinical guidelines in India – a pragmatic approach.
Blog: Towards national standard treatment guidelines (STGs) for India
Blog: Improving detection and control of hypertension in Kerala, India
News
Improving care quality to save mothers’ lives in Kerala
The State Government of Kerala, with technical assistance from iDSI, is now planning to implement quality standards (QS) which are measurable prioritised recommendations from the STG on hypertension. The QS aims to identify undetected cases of hypertension through opportunistic blood pressure measurement for adults visiting a primary healthcare centre. It also aims to improve blood pressure control in known patients. The State Government of Kerala will be integrating indicators for hypertension into the Aadhar linked electronic medical records.
Impact
The quality standards now form the basis of all midwifery emergency training across Kerala.
Implementing two quality standards in all facilities in Kerala, where approximately 500,000 babies are born every year, could result in between 23 and 91 mothers’ lives saved each year through improved prevention and management of post-partum haemorrhage and hypertension in pregnancy.
Implementing these standards is projected to reduce the maternal mortality ratio (MMR) in Kerala by 25%, to around 50 deaths per 100,000, far lower than the national average for India of 130 per 100,000. Since implementing the quality standards, MMR has dropped from 61 (2011-13) to 46 (2014-2016), of which this project has been referenced in national press, as having a “significant difference in delivery care practices”.
Key reads
iDSI resources
- Vlad I, Paily V, Sadanandan R et al.Improving quality for maternal care – a case study from Kerala, India [version 1; referees: 3 approved]. F1000Research 2016, 5:166
(https://doi.org/10.12688/f1000research.7893.1) - Blog https://idsihealth.org/blog/quality-standards-for-maternal-care/
External resources
- Improving Maternal Care in Kerala. Quality Standards. First Edition. http://www.arogyakeralam.gov.in/docs/quality/Quality_standards_for_postpartum_haemorrhage_and_hypertensive_disorders_of_pregnancy_1.pdf
News
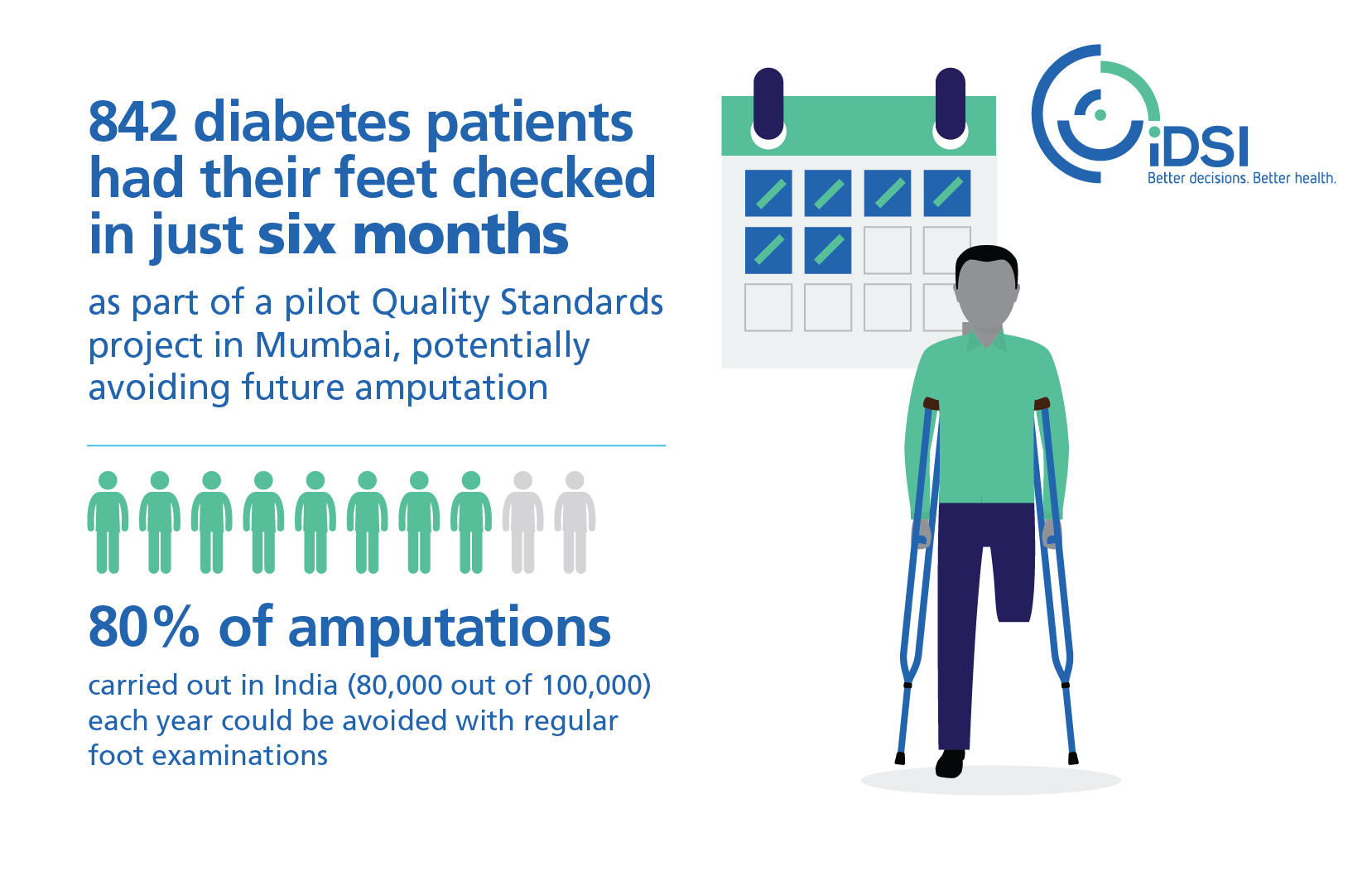
Improving foot care for diabetic patients in Mumbai
The iDSI team reviewed current practice for diabetic feet: physicians, general surgeons and podiatrists all manage patients with diabetic feet, however their responsibilities or management protocols are not clearly defined and foot assessment is not part of routine practice. The team also identified gaps in training and data collection. iDSI guided the BARC diabetes team on: evidence-based efficient ways to run the diabetic foot care clinic as an elective service; data input and patient consent, including providing additional training from UK-based diabetologists on how best to check diabetic feet.
Impact
Prior to the pilot, none of the diabetes patients attending BARC primary health centres had their feet examined, but between April-October 2017 nearly all (93%, 842) diabetes patients received foot examinations by physicians who had received the training. This is a dramatic outcome given how it is normally very difficult to change clinical practice. If all primary health centres in India were to implement the QS to the same high level, this could help to prevent as many as 74,400 amputations per year.
Key reads
- Mishra, S.C., Chhatbar, K.C., Kashikar, A., Mehndiratta, A. Diabetic foot. BMJ 2017, 359, j5064 https://www.bmj.com/content/359/bmj.j5064
The Value of Investing in Cost Data—Lessons from Health Systems Costing Repository in India
10.03
Costing Health Services in India – Incremental Steps Towards More Transparent Decision-Making
10.07
Webinar on iDSI and Healthcare Priority Setting in India
20.05